Osteoporosis in degenerative parkinsonism
Neurodegenerative Parkinsonism comprises a heterogenous group of conditions, including idiopathic Parkinson’s disease (PD), which affects 1 – 2 percent of the UK population aged over 65[1], and atypical Parkinsonian syndromes. Atypical Parkinsonian syndromes include: Progressive Supranuclear Palsy (PSP), Multiple System Atrophy (MSA), Corticobasal Degeneration (CBD) and Dementia with Lewy Bodies (DLB)[2]. Degeneration of dopaminergic neurones within the substantia nigra is the hallmark of PD[3] and the resultant clinical quartet is classically characterised by bradykinesia, rigidity, a resting tremor and postural instability[4].
Falls occur frequently in neurodegenerative Parkinsonism and are associated with an excess risk of fracture, corresponding morbidity, impaired quality of life and increased mortality[5]. 90 percent of fractures are preceded by a fall[6] and falls occur with increased frequency in neurodegenerative Parkinsonism with disease progression; thus, a falls assessment is pivotal in any movement disorders clinic (MDS).
The increased frequency of falls observed amongst patients with PD has been substantiated by the literature, with studies demonstrating falls in up to 50 percent of PD patients over a three month period[7]. Furthermore, in a study of 100 PD patients, 38 patients reported falls with 13 patients falling more than once weekly[8]. Amongst a cohort of 782 pathologically confirmed cases of neurodegenerative Parkinsonism obtained from the Queen’s Square Brain Bank, Williams et al. (2006)[9] demonstrated that falls and fractures occurred in 77.5 percent and 17.1 percent, respectively. Symmetrical disease onset, female gender, postural instability and autonomic dysfunction with orthostatic hypotension independently predicted early falls[9].
A multitude of other factors render this subset of patients susceptible to falls, including: polypharmacy, motor fluctuations, physical deconditioning, reduced bone mineral density and cognitive impairment[1, 10]. Indeed, a community based study evaluating falls risk amongst a cohort of patients with PD, demonstrated that their risk of falls was augmented by motor fluctuations and dyskinesia[11]. Early falls are a cardinal feature of atypical Parkinsonian syndromes, such as PSP and MSA, as opposed to idiopathic PD, in which falls do not tend to occur early in the disease course[9]. In CBD and DLB, gait imbalance and altered mentation are additional significant predisposing factors, whereas in MSA, early orthostatic hypotension, Parkinsonism or cerebellar dysfunction, render patients more susceptible to falls.
In addition to a greater frequency of falls, osteoporosis, defined as a reduced bone mineral density (BMD)[12], is more prevalent amongst patients with PD. The aetiology of osteoporosis itself is multifactorial with numerous modifiable and non-modifiable predisposing risk factors, ranging from age, body mass index (BMI), alcohol excess and hypovitaminosis D[9]. PD, however, constitutes an independent risk factor for osteoporosis and associated fractures[13, 14]. Indeed, the Global Longitudinal Study of Osteoporosis in Women (GLOW) established that PD constitutes the most significant contributor to fracture risk in contrast to the other studied risk factors[13]. The reduction in BMD in PD may manifest secondary to hypovitaminosis D with secondary hyperparathyroidism, reduced BMI[15], immobility and inadequate oral intake secondary to non-motor symptoms, such as nausea, dysphagia and depression[14]. In PD patients, this observed reduction in bone mineral density is most marked within the lumbar spine and femoral neck[1].
Dopaminergic therapy itself – a mainstay of treatment in PD – constitutes an independent risk factor for reduced BMD and associated fractures, the risk of which may be dose-dependent[16, 17]. Dopaminergic therapy frequently alleviates bradykinesia and rigidity, without significantly ameliorating postural instability- patients are therefore more mobile, but still have postural instability rendering them susceptible to falls. This susceptibility may be compounded by levodopa side effects, including excessive daytime somnolence, orthostatic hypotension and visual hallucinations[16].
Depression is more prevalent amongst patents with PD, with major depressive disorder and minor depression affecting 17 and 22 percent of PD patients, respectively[17]. Antidepressant therapy, moreover, predispose patients to osteoporosis, conferring a three to fivefold increased risk of hip and femoral fractures[16, 17, 19]. It is postulated that antidepressants increase the likelihood of falls, and, through serotonin reuptake inhibition, antidepressants negatively impact upon the micro-architecture of bone, increasing the risk of fracture[19, 20]. Research has suggested a dose-dependent effect for selective serotonin re-uptake inhibitors (SSRIs), but not tricyclic antidepressants (TCAs), but with both drug subtypes, the risk of osteoporosis correlates positively with the extent to which serotonin transport systems are inhibited[20]. Dementia, additionally, predisposes patients to both falls[21] and fracture[22], and may occur concurrently with depression in advanced PD.
Reducing the risk of falls is fundamental in reducing associated morbidity and maintaining quality of life. To facilitate this, NICE recommends that patients are assessed with either FRAX or QFracture algorithms to establish fracture risk and determine the need for formal BMD measurement using dual energy X-ray absorptiometry (DEXA) scanning[12]. This thereby ensures that all patents with osteoporosis are reliably identified and commenced on appropriate treatment to reduce their risk of associated fracture.
Whilst the World Health Organization (WHO) FRAX tool is a validated algorithm to establish ten year fracture risk, it does not consider numerous risk factors for osteoporosis, including falls risk and relevant co-morbidities predisposing to osteoporosis and fractures[23]. This subsequently limits its benefit if implemented in evaluating fracture risk amongst patients with neurodegenerative Parkinsonism, many of whom have concurrent depression, cognitive impairment, dopaminergic therapy and antidepressant use. The QFracture algorithm, conversely, incorporates additional variables together with those used in the FRAX tool known to contribute to fracture risk, including falls, care home residence, comorbidities and pharmacotherapy specifically associated with osteoporosis, to more reliably and comprehensively determine fracture risk[24].
The current body of literature on the prevention and management of osteoporosis in PD is limited, however. Moreover, presently there are no randomised trials evaluating osteoporosis risk and management in atypical Parkinsonian conditions. Management approaches are therefore based upon treating general osteoporosis and have been extrapolated for use in neurodegenerative parkinsonism. Nonetheless, management adopts a holistic approach, encompassing both lifestyle and pharmacological measures to reduce falls and subsequent fracture risk. Non-pharmacological measures may be implemented, including falls education, dietary and smoking cessation advice, regular medication reviews to prevent polypharmacy, physiotherapy and occupational therapy, visual assessment and exercise programmes designed to ameliorate strength, balance and flexibility1. Pharmacological interventions, by contrast, include calcium and vitamin D supplementation, bisphosphonate therapy, oestrogen and testosterone supplementation, strontium, raloxifene, and the use of denosumab, a receptor activator of nuclear factor kappa-Β ligand (RANKL) inhibitor[9, 11].
Given the progressive nature of degenerative parkinsonism, falls increase with more prolonged disease duration. Moreover, as discussed above, PD itself, its treatment and associated conditions, such as depression and dementia, predispose patients to osteoporosis, significant fracture risk and associated morbidity. Despite this, however, a bone health assessment is not routinely performed in clinic. Furthermore, whilst PD constitutes both an independent and one of the most significant risk factors for osteoporosis, the risk of osteoporosis in atypical Parkinsonian conditions is less clearly defined. Moreover, few studies evaluate the implementation of appropriate measures to prevent and treat osteoporosis in these conditions.
Objectives
This study aims to 1) characterise the demographic and clinical features of a patient cohort with degenerative Parkinsonism; 2) establish the incidence of comorbid conditions which may contribute to osteoporosis and fracture risk; 3) and evaluate the ten year fracture risk amongst our patient cohort; 4) the need for DEXA imaging and 5) the requirement for treatment with calcium and D for osteoporosis.
Methodology
150 patients attending MDS clinics in central and south Glasgow were identified via Trackcare. All included patients had a diagnosis of degenerative Parkinsonism, specifically idiopathic PD, MSA, PSP, CBD or DLB. As delineated in Figure 1, 50 patients were excluded from the analysis on the following grounds: 34 patients had a diagnosis other than degenerative parkinsonism, including essential tremor or functional movement disorders and two patients were undergoing investigation to establish a definitive diagnosis. Other reasons for exclusion included treatment with bisphosphonates (n = 6); failure to attend clinic (n = 1); incorrect appointment to the MDS clinic (n = 1) and previous analysis of records in this study (n = 6).
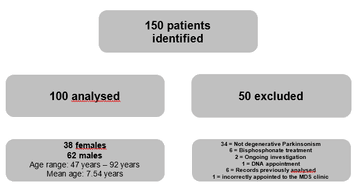
Figure 1: study flow chart outlining the included and excluded patients and establishing the grounds for exclusion
Results
Of the 100 patients analysed, 38 were female and 62 male. Ages ranged from 47 to 92 years, with a mean age of 75.7 years. As displayed in Figure 2, 87 percent of the patient cohort had a clinical diagnosis of confirmed or likely idiopathic PD. The remaining patients were diagnosed with either undifferentiated degenerative Parkinsonism (n = 7), MSA (n = 1), PSP (n = 4), or PSP / IPD (n = 1).
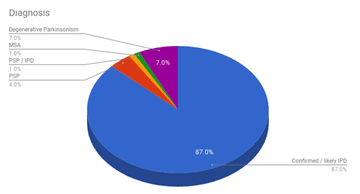
Figure 2: chart displaying the incidence of idiopathic Parkinson’s disease and Parkinson Plus conditions in our patient cohort
Our patent cohort was analysed for co-morbidities predisposing to osteoporosis, the incidence of which is demonstrated in Figure 3. Of the 100 analysed
patients, 22 had a confirmed diagnosis of dementia. Although 11 patients had a confirmed malignancy, several patients were undergoing investigations to evaluate for possible neoplastic disease, but did not have a definitive diagnosis at the time of the analysis. 11 had respiratory disease, including chronic obstructive airways disease, asthma and bronchiectasis; 12 had diabetes mellitus; and 23 had ischaemic heart disease, a previous transient ischaemic or cerebrovascular disease. Two patients had chronic liver disease, two had chronic kidney disease stage four or five (CKD), three had rheumatoid arthritis or systematic lupus erythematosus, one had malabsorptive disease, and seven had endocrine disease (hypothyroidism).
A number of patients within the cohort had also received pharmacotherapy known to predispose to osteoporosis: two patients were maintained on anti-epileptic drugs for epilepsy; 39 were treated with antidepressants, 8 had received steroid therapy and 1 had received hormone replacement therapy. Of the 100 patients analysed, 13 had a known diagnosis of osteoporosis.
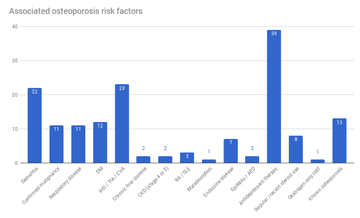
Figure 3: graph demonstrating the incidence of conditions known to predispose to osteoporosis in the patient cohort.
Although 35 percent of the cohort reported recent falls within the six months preceding assessment, a large proportion (52 percent) had no falls documentation in recent clinic letters. The mean 10 year fracture risk, calculated through the QFracture algorithm, was 46.8 percent. Moreover, 68 percent of the cohort had a 10 year fracture risk exceeding 10 percent, thus necessitating DEXA imaging. DEXA imaging, however, was only requested or performed for 20 percent of the cohort. Of the 78 patients in whom bone protection was indicated, only 23 percent had calcium and vitamin D prescribed.
Discussion
This study characterised the demographic and clinical features of a cohort of 100 patients with degenerative Parkinsonism attending central and south Glasgow Movement Disorder clinics. Amongst our cohort, the 10 year fracture risk exceeded 10 percent in 68 percent of patients. This study has therefore corroborated the high incidence of falls and the high fracture risk observed amongst patients with idiopathic Parkinson’s disease, a finding that has been consistently demonstrated in the literature. Beyond this, however, it has elucidated a high fracture risk amongst patents with other subtypes of degenerative Parkinsonism in addition to IPD, a finding that is not well documented in the literature but frequently extrapolated in clinical practice given its association with IPD.
Given the frequency of falls and high fracture risk, this study has emphasised the need for further diagnostic testing in patients with neurodegenerative Parkinsonism when the QFRAX algorithm identifies a fracture risk exceeding 10 percent. As evidenced in our cohort, however, DEXA imaging was not frequently requested or performed when indicated: although 68 percent had a fracture risk exceeding ten percent necessitating imaging, only 20 percent had a DEXA requested or performed. In addition to identifying a requirement for further investigation with DEXA imaging, our study has also highlighted the need for improved bone protection. 55 percent of the patient cohort did not have calcium or vitamin D supplementation or bisphosphonate therapy, although indicated.
One of the strengths of this study is the large sample size, comprising 100 patients with neurodegenerative Parkinsonism. The large patient cohort enhances the statistical power and generalisability of the findings. Furthermore, the use of the more comprehensive QFracture algorithm as opposed to the FRAX algorithm to evaluate fracture risk ensured that relevant conditions predisposing to osteoporosis and demographic features (such as care home residence) were considered and incorporated into the analysis. This will thereby maximise the accuracy and reliability of the fracture risk calculated.
Our study is limited in several respects. Firstly, by its retrospective study design, which has a high reliance on the accuracy of clinical records. Given the retrospective design, integral clinical features known to contribute to fracture risk were often not well documented, such as body mass index (BMI), smoking and alcohol status, and family history of osteoporosis. Thus, the fracture risk calculated in our cohort may under-estimate the actual risk amongst our patients. Secondly, although our study has a large sample size, all patients were recruited from specialist outpatient MDS clinics within central and south Glasgow. Thus, the study population does not reflect the random distribution of prospective population based designs. A multicentre prospective study would perhaps enable an even larger patient cohort to be amassed that is more reflective of the degenerative parkinsonism population across the country. The recruitment of the patient cohort from the same tertiary centre could conversely be construed as a relative strength, however, as it facilitated consistent clinical diagnosis and characterisation.
Although the QFracture algorithm incorporates a more comprehensive range of variables than the FRAX algorithm, it s use in this study nonetheless has several inherent limitations. The algorithm specifically incorporates IPD as a variable given its association with osteoporosis, but our study included patients with undifferentiated degenerative parkinsonism and atypical parkinsonian conditions (MSA, PSP, CBD and DLB) in addition to IPD. Furthermore, the algorithm specifically considers CKD stages 4 and 5 as a significant osteoporosis risk factor, although a number of patients also had CKD stages 1 – 3. With respect to pharmacotherapy with a deleterious effect on bone health, the algorithm considers steroid tablets as significant. Only steroid tablets were considered significant in our analysis to ensure consistency with the algorithm. However, given this, our findings may under-estimate the risk of osteoporosis and fracture in our cohort.
To conclude, despite the aforementioned limitations, this present study corroborates findings in the literature of a high fracture risk amongst patients with degenerative Parkinsonism. It has also highlighted the need for further diagnostic testing through DEXA scanning and the prescription of bone protection, when indicated. Certain integral interventions may be implemented to identify fracture risk earlier and ensure appropriate measures are introduced to prevent falls, fractures and subsequent morbidity. These measures include improved documentation of falls, BMI, smoking and alcohol status and the completion of a pre-clinic QFracture score, prior to all clinic assessments. This would reliably highlight patients with a fracture risk exceeding 10 percent to ensure that they are referred for a DEXA and appropriately prescribed bone protection when indicated.
References
- Torsney KM, Noyce AJ, Doherty KM, Bestwick JP, Dobson R, Lees AJ. Bone health in Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry [Internet]. 2014;1–8. Available from: http://www.ncbi.nlm.nih.gov/pu...
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Vol. 18, Movement Disorders. 2003. p. 467–86.
- Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Vol. 39, Neuron. 2003. p. 889–909.
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry [Internet]. 2008;79(4):368–76. Available from: http://www.ncbi.nlm.nih.gov/pu...
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of Gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Vol. 19, Movement Disorders. 2004. p. 871–84.
- Cummings SR, Melton LJ. Osteoporosis I: Epidemiology and outcomes of osteoporotic fractures. Vol. 359, Lancet. 2002. p. 1761–7.
- Pickering RM, Grimbergen YAM, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22(13):1892–900.
- Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson’s disease 31. Vol. 12, Clin.Neuropharmacol. 1989. p. 98–105.
- Williams DR. Predictors of falls and fractures in bradykinetic rigid syndromes: a retrospective study. J Neurol Neurosurg Psychiatry [Internet]. 2006;77(4):468–73. Available from: http://jnnp.bmj.com/cgi/doi/10...
- Bezza A, Ouzzif Z, Naji H, Achemlal L, Mounach A, Nouijai M, et al. Prevalence and risk factors of osteoporosis in patients with Parkinson’s disease. Rheumatol Int [Internet]. 2008;28(12):1205–9.
- Schrag a, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123 ( Pt 1:2297–305.
- NICE. Osteoporosis : fragility fracture risk. Natl Clin Guid Cent. 2012;Short clin(August).
- Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone. 2012;50(6):1288–93.
- Dobson R, Yarnall A, John Noyce A, Giovannoni G. Bone health in chronic neurological diseases: a focus on multiple sclerosis and parkinsonian syndromes. Pr Neurol [Internet]. 2013;13:70–9. Available from: http://dx.doi.org/10.1136/
- Vaserman N. Parkinson’s disease and osteoporosis. Vol. 72, Joint Bone Spine. 2005. p. 484–8.
- Arbouw MEL, Movig KLL, Van Staa TP, Egberts ACG, Souverein PC, De Vries F. Dopaminergic drugs and the risk of hip or femur fracture: A population-based case-control study. Osteoporos Int. 2011;22(7):2197–204.
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with parkinsonism and anti-Parkinson drugs. Calcif Tissue Int. 2007;81(3):153–61.
- Reijnders JS a M, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord [Internet]. 2008;23(2):183–9; quiz 313. Available from: http://www.ncbi.nlm.nih.gov/pu...
- Lieberman A. Depression in Parkinson’s disease — a review. Acta Neurol Scand [Internet]. 2006;113(1):1–8. Available from: pm:16367891
- Van Den Brand MWM, Samson MM, Pouwels S, Van Staa TP, Thio B, Cooper C, et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos Int. 2009;20(10):1705–13.
- Wood BH. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry [Internet]. 2002;72(6):721–5. Available from: http://jnnp.bmj.com/cgi/doi/10...
- Melton LJ, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Oberg AL, et al. Fracture risk after the diagnosis of Parkinson’s disease: Influence of concomitant dementia. Mov Disord. 2006;21(9):1361–7.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAXTM and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97.
- Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ [Internet]. 2012;344(may22 1):e3427–e3427. Available from: http://www.bmj.com/cgi/doi/10....
More Parkinson's Academy Falls & bone health Projects
'The things you can't get from the books'
Parkinson's Academy, our original and longest running Academy, houses 20 years of inspirational projects, resources, and evidence for improving outcomes for people with Parkinson's. Led by co-founder and educational director Dr Peter Fletcher, the Academy has a truly collegiate feel and prides itself on delivering 'the things you can't get from books' - a practical learning model which inspires all Neurology Academy courses.